NS001 Naloxone:
Rapid Reversal of Opioid Overdose
NS001, Nasus Pharma’s intranasal powder formulation of naloxone, is designed to rapidly reverse opioid overdoses—a global public health crisis. Backed by a completed phase 3 pivotal study (n=42), NS001 validates the Nasax® powder platform, demonstrating faster absorption and higher systemic levels compared with Narcan®.

The Medical Need
Opioid overdose is a leading cause of accidental death worldwide
Current rescue therapies face challenges with speed, consistency, and administration under duress
A powder-based alternative ensures needle-free rapid delivery that may be advantageous for high-potency opioids
Pivotal Study Validated the Superiority of Powder over Liquid, Demonstrating Nasax Platform Delivers Naloxone Faster Compared to Narcan®
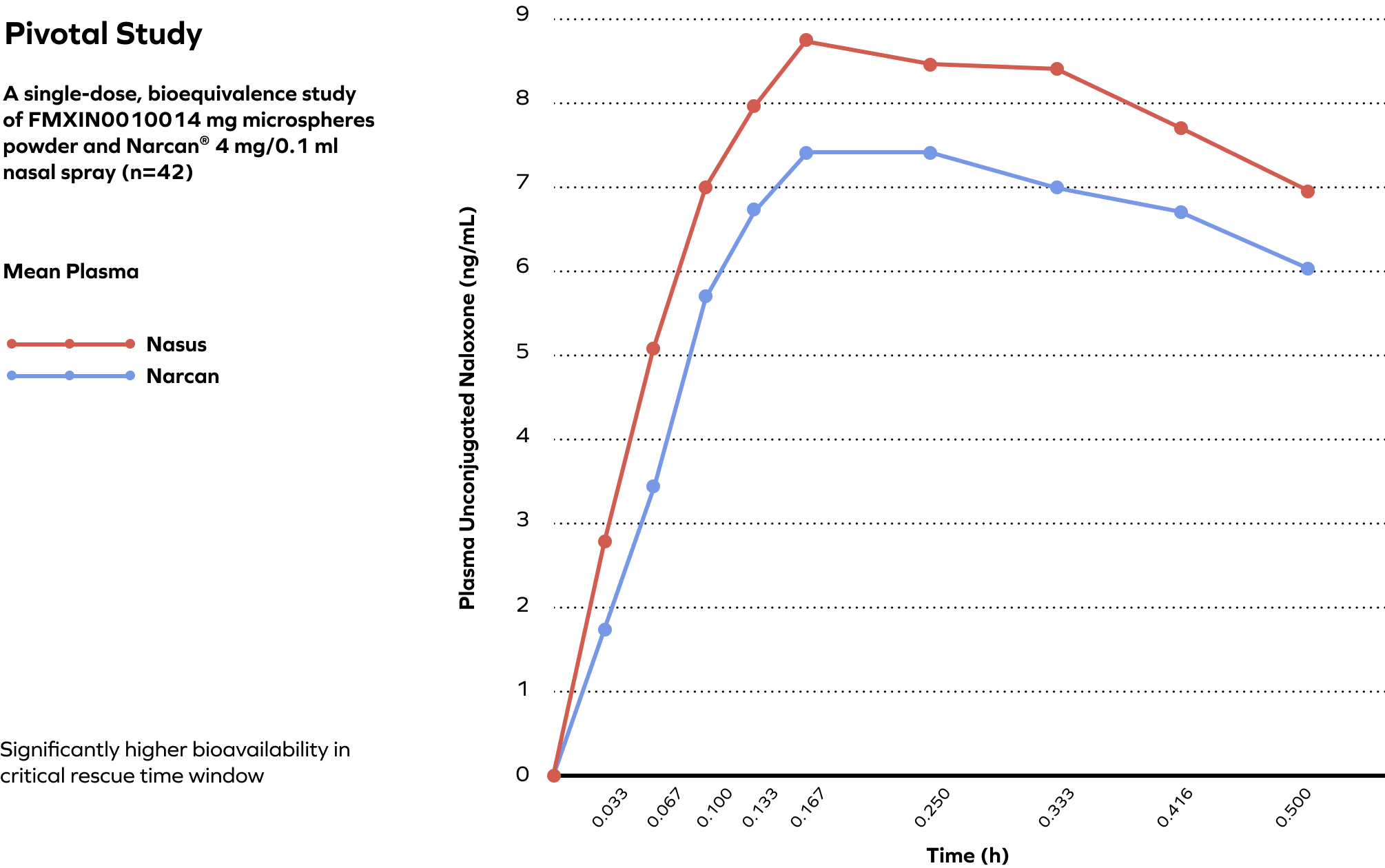
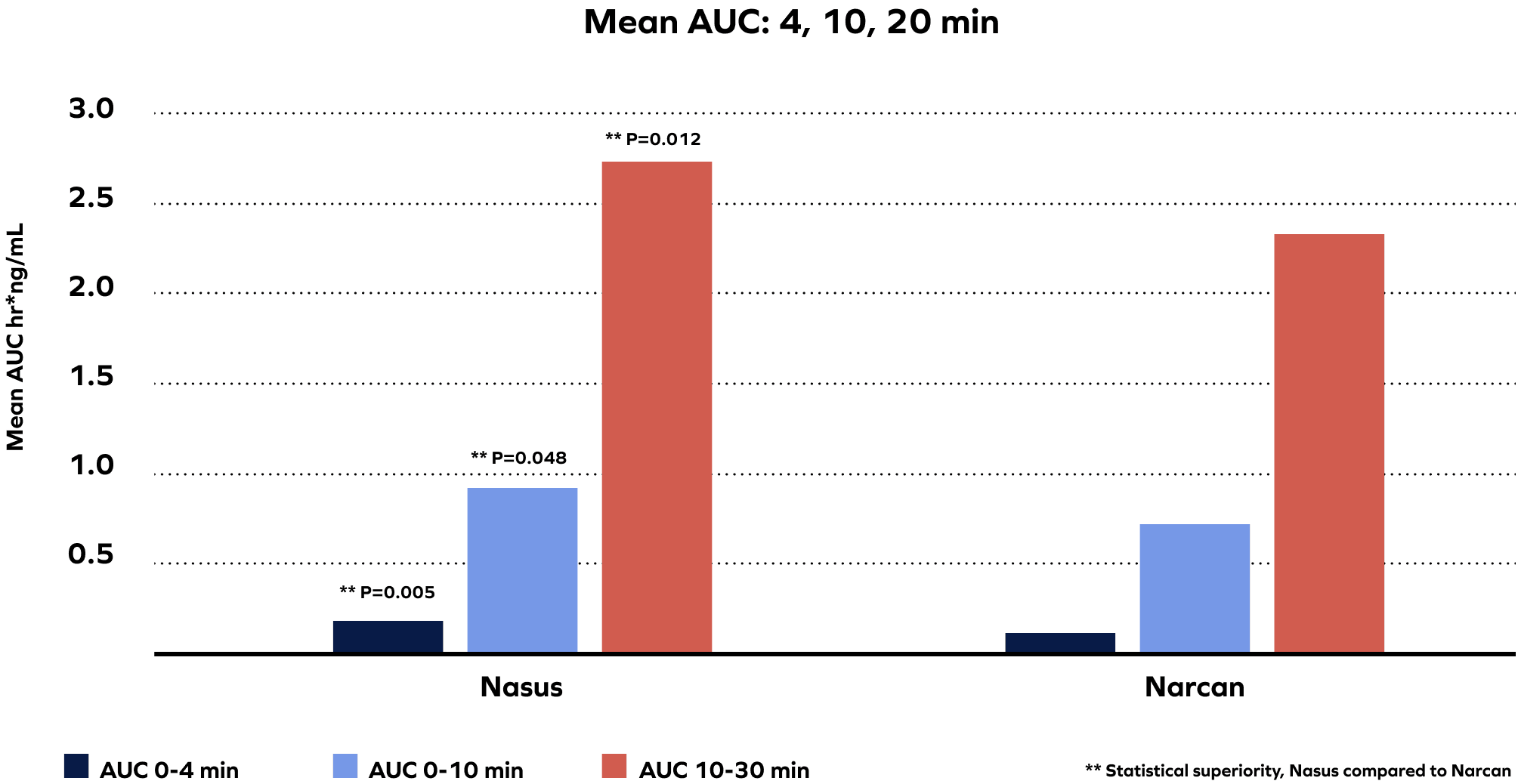
In our phase 3 (n=42) intranasal naloxone study (NS001), our formulation provided faster delivery and higher mean absorption of naloxone compared to Narcan®
NS001 is available for partnering
